The Food and Drug Administration (FDA) highlights our recent innovation, the MSA Advantage® 290 Elastomeric Respirator, as a solution for healthcare personnel
-
 Air-Purifying Respirators
Air-Purifying Respirators
-
 Supplied-Air Respirators
Supplied-Air Respirators
With the Covid-19 pandemic, access to personal protective equipment (PPE) for frontline workers has continued to be a significant barrier. Understanding the challenge, MSA introduced a first-of-its-kind elastomeric respirator, without an exhalation valve. MSA's new Advantage 290 half-mask respirator, with source control, provides healthcare professionals a new, cost effective option to help keep them safe while working to save lives.
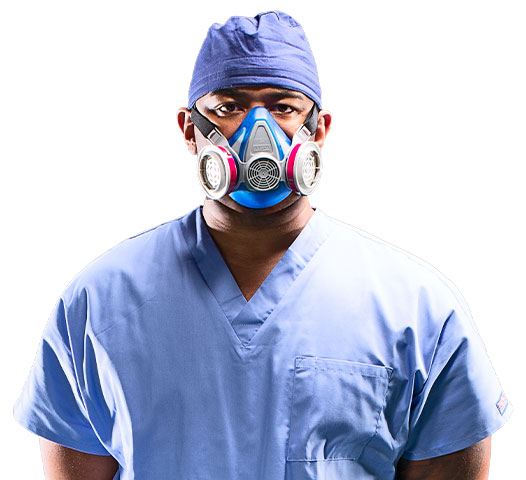
Elastomeric respirators are designed to be reusable but had limits in their use in a healthcare environment because released air from the exhalation valve can compromise sterile fields, like operating rooms.
The National Institute for Occupational Safety and Health (NIOSH) recently approved the MSA Advantage 290 Elastomeric Respirator, the first to provide both wearer and source control, making it suitable for situations where a sterile field is required.
Source control - which means preventing the transmission of infection through a wearer's respiratory droplets that can come from speaking, coughing, or sneezing - is achieved by the elimination of the exhalation valve. The Advantage 290 filters and contains that exhaled breath at its source, so that it does not spread to others or contaminate the surrounding environment.
Click to read the article in its entirety in the U.S. Food and Drug Administration (FDA) Newsletter, Voices.